Madhya Pradesh Police Arrest Pharma Firm Owner Linked to Deadly Coldrif Cough Syrup
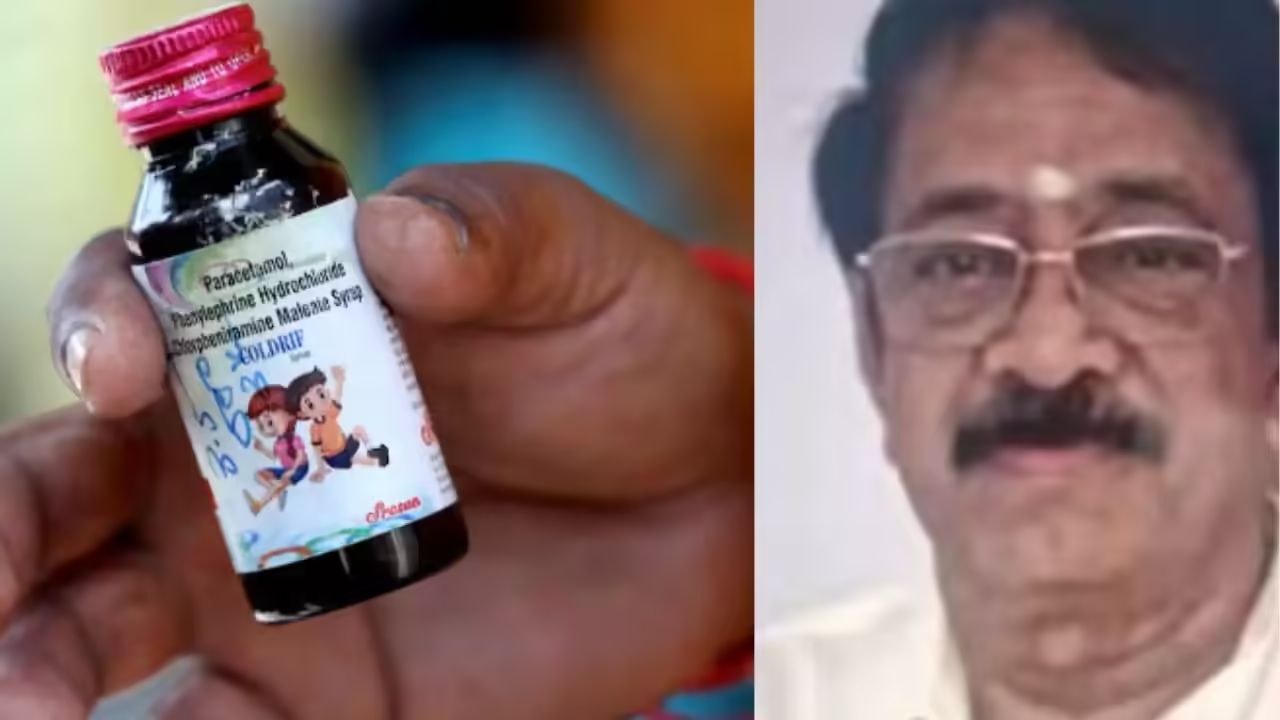
Bhopal, October 9, 2025 Ranganathan Govindan, the owner of Sresan Pharmaceuticals and the key accused in the deadly Coldrif cough syrup case, was arrested by Madhya Pradesh police early Thursday morning following a meticulously planned operation. The cough syrup manufactured by his company has been linked to the deaths of at least 20 children in Chhindwara and caused serious illness in several others due to the presence of a highly toxic substance exceeding permissible limits. Ranganathan and his wife had been absconding since the tragedy came to light, prompting a statewide hunt. Acting on precise intelligence, a special police team from Madhya Pradesh, led by the Sub-Divisional Police Officer of Parasia, tracked the pharmaceutical owner to Chennai. The team, which included female officers, cyber experts, and drug inspectors to handle technical and legal aspects, executed the arrest at around 1:30 am. Following his arrest, Ranganathan was escorted to Sresan Pharma’s Kancheepuram factory, where authorities seized critical documents to aid the ongoing investigation. Police officials are now seeking a transit remand from a Chennai court to bring him to Chhindwara for detailed questioning and further proceedings. Sources confirmed that the operation was the result of careful planning, with investigators tracking Ranganathan’s vehicles, monitoring his residence, and examining his financial transactions. The police also highlighted that a Rs 20,000 reward had been announced a day prior for information leading to his arrest, which ultimately helped expedite the capture. Sresan Pharmaceuticals, based in Chennai, has faced intense scrutiny since the incident. Investigations revealed that the company, originally registered as a private limited firm in 1990, had been struck off by the Ministry of Corporate Affairs but continued operations under a proprietary structure. The firm, which claimed to manufacture and trade in syrups, tonics, and herbal formulations, is now facing allegations of gross negligence, regulatory violations, and failure to adhere to drug manufacturing standards. The tragic incident has raised serious questions about regulatory oversight and the functioning of pharmaceutical operations in India. Coldrif syrup, which contained banned chemical ingredients, reached unsuspecting children, causing widespread alarm and outrage among the public and authorities. Authorities are also examining how the supply chain—from chemical suppliers to stockists and medical representatives—allowed the toxic syrup to enter the market unchecked. Police sources stated that the investigation will now expand to cover every link in the distribution network, including suppliers and distributors, to hold all responsible parties accountable. The arrest of Ranganathan is expected to provide crucial leads that may help uncover the full scope of negligence and criminal activity behind one of the most horrifying pharmaceutical tragedies in recent Indian history. Authorities continue to urge the public and healthcare professionals to remain vigilant, report any irregularities in drug supplies, and cooperate with the investigation. The swift action and coordinated efforts of Madhya Pradesh police reflect the urgency to bring justice for the victims and ensure accountability within the pharmaceutical sector. As the probe deepens, officials aim to implement stricter oversight mechanisms to prevent such incidents in the future, safeguarding children and the wider population from similar tragedies. Madhya Pradesh Police Arrest Owner of Deadly Coldrif Cough Syrup Ranganathan Govindan, owner of Sresan Pharmaceuticals, was arrested in Chennai early Thursday for his role in the Coldrif cough syrup tragedy that claimed at least 20 children’s lives in Chhindwara. The syrup contained a highly toxic substance exceeding safe limits. Ranganathan and his wife had been on the run since the incident surfaced. A special police team tracked him using intelligence, vehicle monitoring, and financial records. Authorities seized crucial documents from his Kancheepuram factory and plan to bring him to Chhindwara for interrogation. The investigation will now examine the entire supply chain to identify all responsible parties.




